Custom Affinity Resin Development
Industry-best custom affinity resin development from Repligen offers unparalleled ligand diversity, streamlined process development and proven success across a broad array of biomolecules.
Streamlined development process
- 3 months to custom affinity solution
- Available pre-packed in OPUS® Columns
安全なサプライ
- Redundant manufacturing at 3+ sites
- Worlds largest ligand manufacturer at 4+ sites
- 10+ year supply agreements

 SPEED TO MARKET
SPEED TO MARKET
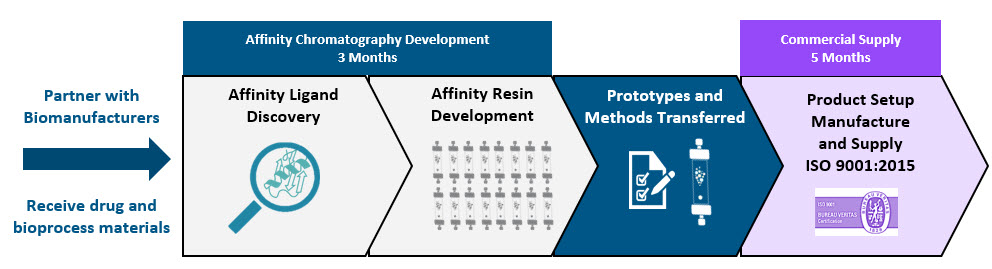
Streamlined custom affinity resin development process
- 100% ligand discovery success rate
- 3 months to prototype affinity resins
- 5 months to manufacture and supply resin for use in GMP operations
- Unrivaled timeline and delivery success rate
Set resin specifications, materials transfer
1-2 weeks
With a focus on speed and recognition that each of our partners’ needs is unique, we begin our collaborations by launching a thorough situation analysis that defines specifications, determines timelines, and sets strategy to achieve optimal results.
Perform AVIPure® affinity ligand discovery
6 weeks
In simplistic terms, high affinity, high selectivity affinity ligands can be thought of as a lock-and-key model for biological recognition. Given the diversity across biologics, it is not possible to predict a priori which ligand type is better than others. However, it is known that a single ligand class or scaffold will not work every time. The cost of time and failing to develop a solution is unrecoverable.
To avoid this, Avitide applies its proprietary portfolio of AVIPure® ligand scaffolds (50+ to date) and library diversity (greater than 1E14), enabling a vast interrogation of different ligand sizes, shapes, conformations, and contact areas. The implementation of this extreme diversity enables delivery success rate and unrivaled affinity purification performance in the industry. Throughout ligand discovery stage, we quickly screen and arrive at optimal ligand candidates for each biomolecule focusing on affinity, stability, and manufacturability.
Following analytical characterization of ligand binding, elution kinetics, and selectivity, we deliver a data package to our partners and recommend lead ligands to transition to the resin development stage.

Develop custom affinity resin
6 weeks
Once a subset of ligand candidates is selected, production is scaled up using methods appropriate for commercial resin manufacturing. We then produce hundreds of candidate resins for screening, evaluating a resin manufacturing DOE that takes into consideration ligands and ligand density, chemistry, matrix type (size and porosity), and linkers. We also evaluate affinity resin separation performance in relevant bioprocess feedstreams, establishing binding and elution conditions within each molecule’s stability profile and separation and operational conditions that achieve each of our partner’s resin specifications.
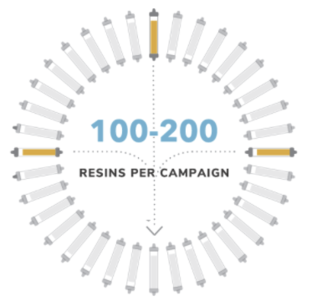
Receive prototype resins, nominate lead resin
1-2 months
With the project complete, we conduct an onsite technology transfer of the resins, data, and protocols to our partner. We then collaborate, providing ongoing technical support wherever necessary, to ensure operational success. Our partners confirm our results and initiate further testing to nominate their preferred resin for commercial supply.

 SPEED TO MARKET
SPEED TO MARKET
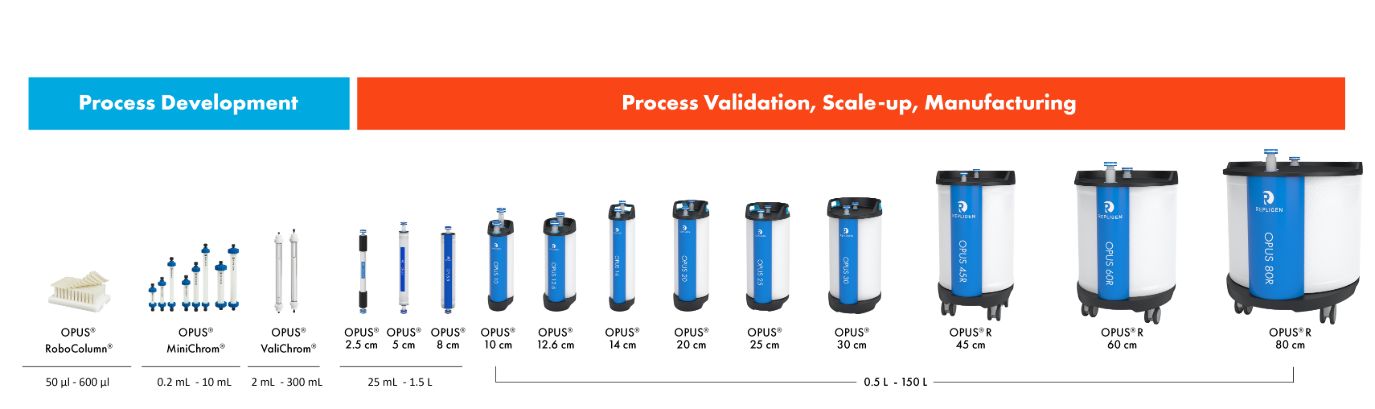
Available pre-packed in OPUS® Columns for quick deployment
Custom affinity resins are available in pre-packed and pre-qualified OPUS® Columns for rapid GMP implementation as well as in loose resin formats.
OPUS® Columns allow you to progress from development to manufacturing scale in weeks, using pre-packed and pre-qualified chromatography columns.
Manufacturing Centers of Excellence
Repligenは、ISO 9001品質管理システムに基づいて、バイオ医薬品業界向けの製品を開発・製造しています。高品質で安定した、堅牢な製品をタイムリーに提供し、お客様の事業継続性を保証することを重視しています。
Repligen manufacturing sites are located in Massachusetts, California, and New Jersey in the United States and in Sweden, France, The Netherlands, Germany and Estonia.


お客様第一。
サポートは、Repligenという企業の遺伝子に組み込まれています。弊社の目標は、卓越した顧客体験を提供すること、そしてRepligenの製品やサービスの適用や導入を効率よく成功に導くためにサポートすることです。
- Field Application Support
- カスタマーサービス
- フィールドサービスエンジニア

