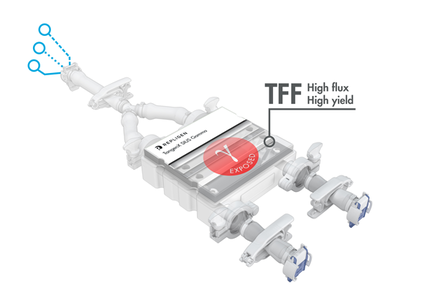
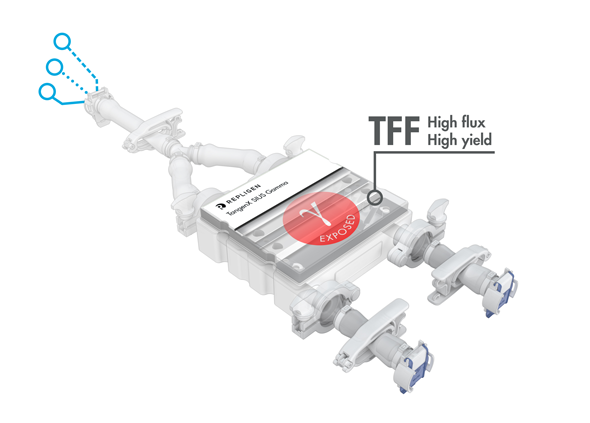
TangenX® SIUS® Gamma TFF Devices
With the performance and efficiency of SIUS® Cassettes, SIUS Gamma Devices come as a convenient, fully assembled, closed, and sterile system.
Achieve higher flux, closed device and connectivity freedom
TangenX® SIUS® Gamma TFF Devices embody the performance and efficiency of SIUS® Cassettes in a convenient, fully assembled, closed, and sterile system. Acclaimed performance with up to 30% flux increase over conventional membranes, connectivity freedom, and a library of configurations, converge into one, simple TFF solution. SIUS Gamma TFF Devices are ideal for gene therapy applications, bioburden-sensitive operations, and hazardous processes.
 READY-TO-USE
READY-TO-USE
Clamp-and-go connectivity
The diversity of connectivity options makes building closed systems a challenge. Genderless AseptiQuik® connectors featured on TangenX® SIUS® Gamma TFF Devices make life easier. Simply clamp the SIUS® Gamma TFF Device into a holder, connect to a flow path, and go. It's that easy.
Click on the links below to explore how to assemble SIUS Gamma TFF Devices. TangenX® SIUS® hardware may be purchased separately.
 READY-TO-USE
READY-TO-USE
Simple connectivity with ProConnex® Flow Paths
Custom ProConnex® Flow Paths complete your TFF solution. Flow paths are build using Class VI Bioprocess-grade materials that are BSE/TSE-free and Lot-traceable.
CONFIGURED FOR YOU
Build a custom flow path to your specifications
- Engineer-designed
- 250以上のコンポーネントから選択可能
- 接続
- Process reservoirs
- 圧力変換器
- 無菌コネクタ
READY TO SHIP
Order a pre-built and quality tested off-the shelf ProConnex® Flow Path stocked for rapid delivery.
- 個別に調査
- 完全性テストが可能
- クリーンルーム組立
- 二重包装
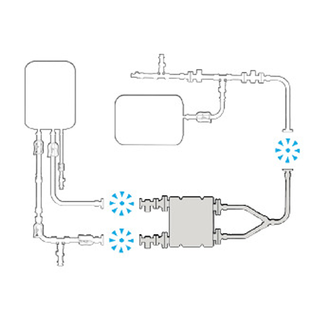
PLUG INTO EXISTING
ジェンダーレスコネクターの柔軟性を利用して、既存のフローパスにシームレスに接続します。
 HIGH PERFORMANCE
HIGH PERFORMANCE
同等の性能をすぐに利用できる利便性
Transition from SIUS® Cassettes to SIUS® Gamma TFF Devices seamlessly.
Membrane performance is unaffected by exposure to gamma irradiation.
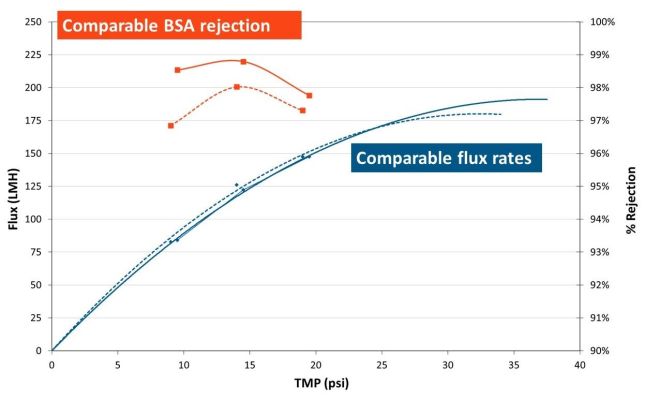
When comparing performance of TangenX® SIUS® Cassettes (solid line) and TangenX® SIUS® Gamma TFF Devices (dashed line):
- Permeate flux is comparable
- BSA rejection varies by less than 1%
 HIGH PERFORMANCE
HIGH PERFORMANCE
Diverse options for diverse process needs
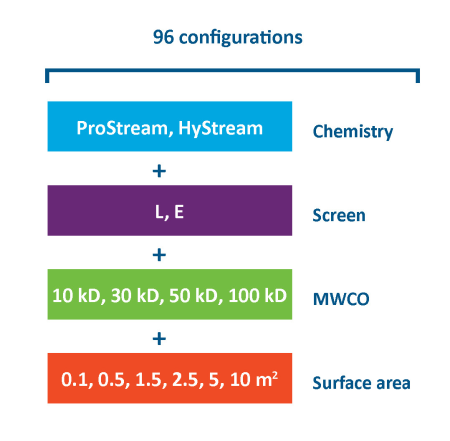
TangenX® SIUS® Gamma TFF Devices are offered with two membrane chemistries, two screen types, a wide selection of molecular weight cutoffs (MWCO), and a broad range of surface areas. With 96 configurations available, TangenX® SIUS® Gamma TFF Devices accommodate diverse process requirements for ultrafiltration and diafiltration applications from process development to commercial manufacturing.
- Neutral charge
- 低いタンパク結合率
- Excellent chemical resistance
- 親水性
- 低いタンパク結合率
- minimal fouling with hydrophobic species
 READY TO SCALE FAST
READY TO SCALE FAST
Scalable from lab to process scale
TangenX® SIUS® Gamma Devices scale from process development to large-scale manufacturing. Molecular weight cut-offs (MWCO)s extend from 10 kD up to 100 kD. Mermbrane area range from 0.1 - 10 m2.

分子量カットオフ
10 kD, 30 kD, 50 kD, 100 kD
Membrane area
0.1, 0.5, 1.5, 2.5, 5, 10 m2
TangenX® SIUS® Gamma Devices
TangenX® SIUS® Gamma TFF Devices incorporate the performance-leading TangenX® TFF membrane and cassette manufacturing technologies into a closed, gamma-irradiated, single-use assembly. Genderless, aseptic connections easily integrate into your current UF/DF flow path.
Click on the links below to explore different features.
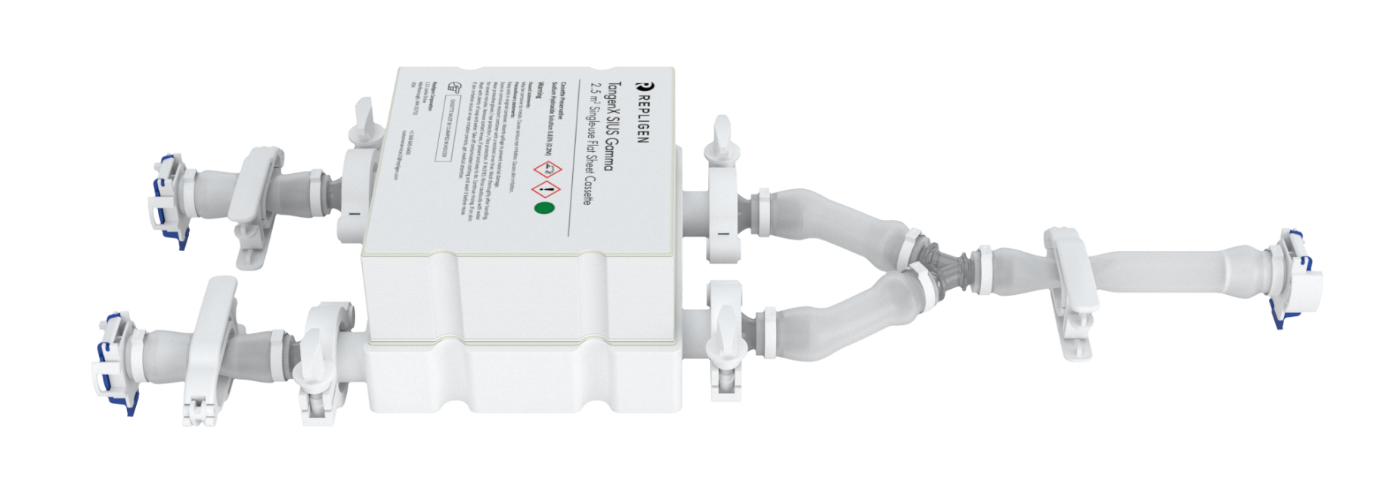


Ohhhh, snap!
In a snap, you can build a closed TangenX® SIUS® Gamma flow path. It’s that easy.
TangenX® SIUS® Gamma TFF Devices incorporate the performance-leading TangenX® TFF membrane and cassette manufacturing technologies into a closed, gamma-irradiated, single-use assembly. Genderless, aseptic connections easily integrate into your current UF/DF flow path.

Manufacturing Centers of Excellence
Repligenは、ISO 9001品質管理システムに基づいて、バイオ医薬品業界向けの製品を開発・製造しています。高品質で安定した、堅牢な製品をタイムリーに提供し、お客様の事業継続性を保証することを重視しています。
Repligen manufacturing sites are located in Massachusetts, California, and New Jersey in the United States and in Sweden, France, The Netherlands, Germany and Estonia.

Validated manufacturing
高品質な製造
TangenX® Cassettes are manufactured in a fully validated and documented manufacturing process according to the principles of cGMP. Each cassette comes with a Quality Assurance Certificate.

包括的なレギュラトリーサポートファイル
Each TangenX® TFF Cassette is supported by a Regulatory Support File (available upon request) that includes:
- 製品情報
- カセットのデザイン
- 素材
- 製品の性能
- 安全に関する情報
- 文書化システム
- 製品の製造
- 資格証明
- 製造プロセスの検証
- リリーステスト


お客様第一。
サポートは、Repligenという企業の遺伝子に組み込まれています。弊社の目標は、卓越した顧客体験を提供すること、そしてRepligenの製品やサービスの適用や導入を効率よく成功に導くためにサポートすることです。
- Field Application Support
- カスタマーサービス
- フィールドサービスエンジニア
リソース
by David Bianchi, Carl Breuning, Michael LaBreck, Shelly Parra, Mary Jo Wojtusik, Repligen Corporation
Alex Meola, Michael Mercaldi, Thomas Thiers, Homology, Medicines Inc.
Repligen Corporation
Homology, Medicines社
2020年7月